Orthotopic Liver Transplantation: Surgical Techniques
Cataldo Doria , Samuel Goldstein and Ignazio R. Marino
Abstract
The authors have chosen to describe the most commonly used surgical techniques for liver transplantation and their variations, namely, the standard technique with and without venous-venous bypass, the piggy-back technique with and without venous-venous bypass, and the Belghiti modification of the piggy-back. In addition to that, two rare forms of liver transplantation will be discussed: the procedures used for situs viscerus inversus and auxiliary liver transplantation.
Introduction
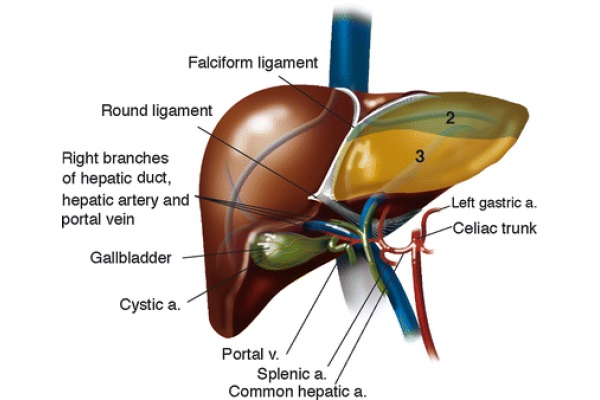
Thomas E. Starzl performed the first orthotopic liver transplantation in 1963 (Starzl 1969). The introduction of venous-venous bypass, complex immunosuppression regimens, and computer imaging contributed to making this procedure widely available in a span of half a century. Figure 1 indicates the textbook hepatic anatomy.
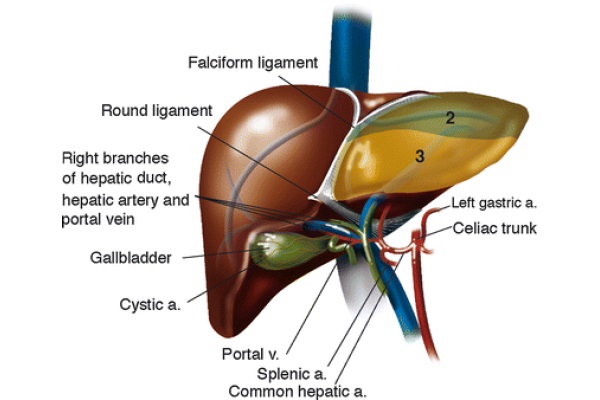
Fig. 1 - Liver anatomy
Orthotopic Liver Transplant (OLT): Standard Technique With and Without Venous-Venous Bypass
The standard procedure of orthotopic liver transplant is the preferred surgical method. This technique should be used any time a liver malignancy is growing next to the inferior vena cava (IVC). The standard technique is the simplest of the procedures to perform, and it takes the shortest amount of time. The introduction of the venous-venous bypass has made the training of many surgeons possible and has dramatically decreased the intraoperative mortality experienced during the early days of liver transplantation. Before the introduction of the venous-venous bypass, survival rates were low in the setting of hemodynamic instability (Shaw et al. 1984). Patients were especially vulnerable during the anhepatic phase due to decreased venous return to the heart and hypertension in portal and systemic vessels upon clamping of the IVC and the portal vein (Shaw et al. 1984). The venous-venous bypass shunts blood flow from the portal and caval systems in the lower part of the body into the internal jugular vein, bypassing the inferior vena cava. This shunt provides the surgical team with time to implant the allograft without subjecting the patient to a decreased venous return to the heart during a complete occlusion of the IVC.
The preferred incision of choice is known as the Mercedes-Benz. It consists of a bilateral, subcostal incision with an upper midline extension made with electrocautery. Once the peritoneum is entered, the ascitic fluid, if present, is drained. Specimens are sent for culture and sensitivity studies. The round ligament of the liver is divided between 0 silk ties, and the falciform ligament of the liver is divided with electrocautery. At this stage, the xiphoid process is removed using electrocautery and heavy scissors. Subsequently, the midline peritoneum is tucked to the fascia with interrupted 2/0 silk stitches.
This maneuver facilitates reopening of the midline if needed. In fact, by bringing the peritoneum up to the fascia, the intensity of the adhesions is limited in that area. In addition to that, covering the stump of the xiphoid process with peritoneum prevents injuries during the mobilization of the cirrhotic liver and the implantation of the allograft. A wet folded lap is placed on the tip of the spleen to avoid splenic injury caused by the retractor’s blades. To provide adequate exposure, the surgeon uses a combination of the self-retaining rib-grip (Stieber) and the Iron Intern retractors. The operation begins with an exploratory laparotomy. This phase is particularly important and aims to rule out possible contraindication to transplantation. Contraindication is most commonly lymph node metastasis from primary liver cancers. Subsequently, the elements of the hepatic hilum are skeletonized. First, the common bile duct is isolated and transected between 2/0 silk ties. A sample of bile is collected and sent for culture. This is the second and last standard culture obtained during liver transplantation. Patients undergoing liver transplantation are often colonized with bacteria that can cause infection postoperatively under the effect of the immunosuppressive treatment. Therefore, knowing which bacteria, if any, are colonizing the bile duct and the peritoneal cavity can help expedite antibiotic treatment while waiting for the final culture results. Next, the hepatic artery is divided between 2/0 silk ties. It is a good practice for the surgeon to alert the anesthesiologist before the silk is tied off on the artery. This provides the anesthesiologist enough time to draw the last arterial blood sample while the native liver is perfused with systemic blood flow. In fact, the lactate clearing activity of the liver is predominantly controlled by the blood flow coming from the hepatic artery. Lastly, the portal vein is skeletonized. At this stage we proceed to dissect the hepatic artery proximally to 1 cm below the takeoff of the gastroduodenal artery (GDA). The distance of 1 cm generally provides enough room to place a surgical clamp on the common hepatic artery and to rotate the vessels when performing the anastomosis.
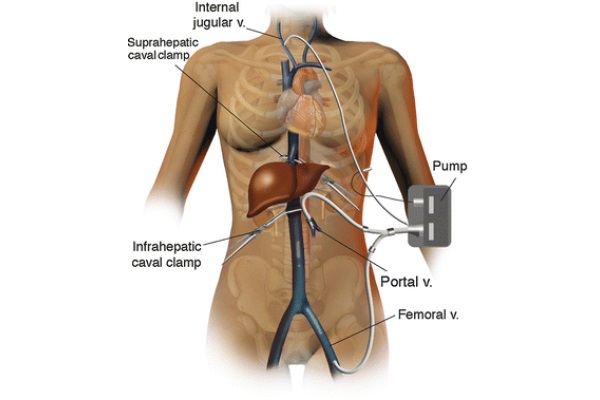
At this stage, the access sites for the venous-venous bypass are prepared. The return cannula is placed in the right internal jugular vein by the anesthesia team after induction of general anesthesia and before prepping the surgical field. The IVC cannula is placed percutaneously through the left groin using a Seldinger technique. Although the left groin is preferred, the right groin can be used if needed. Of note, the IVC is accessed through the iliac-femoral vessels. To safely place the portal vein cannula, the portal vein skeletonization is first maximized to obtain the longest possible vessel trunk. Then, a large surgical clamp is applied distally on the portal vein as far as possible in the porta hepatis, while the proximal end of the vessel is clamped between fingers. The portal vein is divided as close as possible to the clamp in the porta hepatis. To facilitate the cannulation of the portal vein, three tonsils are applied on the edge of the vessel to keep it open. The cannula is secured in place by two wet umbilical tapes. Both cannulas are tested and flushed with a heparinized saline solution. The portal and femoral vein cannulas are connected with a Y connector, and the bypass cannula is connected to the patient, as shown in Fig. 2. The bypass is started and the flow is maintained above 1 l per minute. Below this speed, the patient is subject to thrombosis (Shaw et al. 1984). If the volume flow rate slows to below 1l/min, it may be a sign of hypovolemia or malpositioning of the portal vein cannula. In the first case, administration of fluid boluses can increase the bypass flow rate. At times, low flow results from a cannula facing the wall of one of the vessels, typically at the juncture of the splenic and superior mesenteric veins. To increase the flow in this case, the surgeon must maneuver the cannula away from the wall of the vessel. However, if the flow rate cannot be increased above 1 L/min, the patient is required to go off bypass.
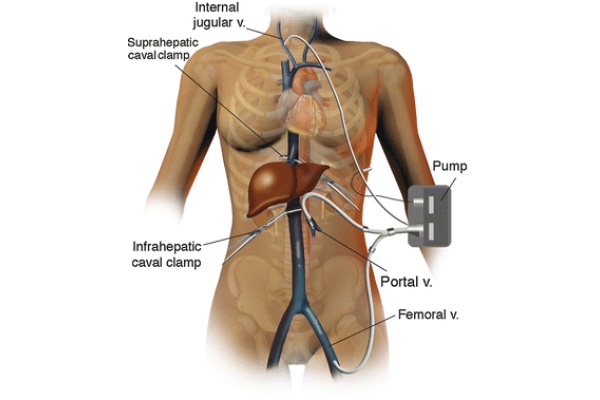
Fig. 2 - Venous-venous bypass anatomy
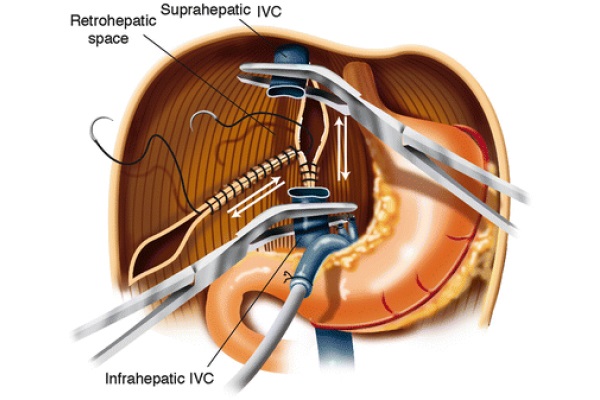
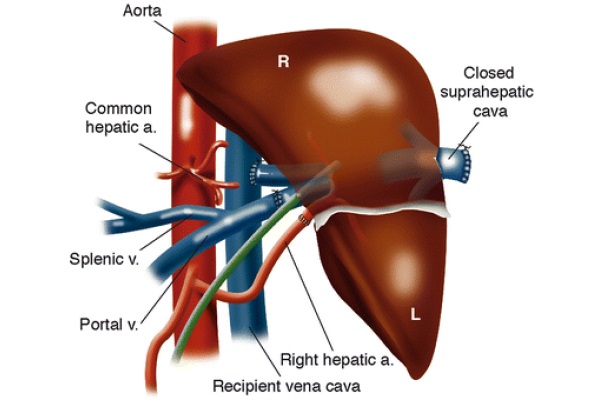
The distal stump of the portal vein is oversewn with Prolene 3/0. The infra-hepatic IVC is skeletonized and cross-clamped with an adult angle Potts clamp. The left triangular ligament is divided with electrocautery, and the gastrohepatic ligament is divided between 2/0 silk ties. The right triangular ligament is divided with electrocautery. The suprahepatic IVC is encircled and clamped with a German clamp. The liver is removed from the field using blunt and sharp dissection. As soon as the native liver is removed from the surgical field, the right adrenal vein is tied off using a 0 silk tie, which is passed around the area where this vessel merges with the IVC. Further hemostasis is obtained in the bare area of the liver by two running 2/0 Prolene sutures: one vertically placed in the IVC area and one from the tip of the right triangular ligament forward as shown in Fig. 3. These two running sutures are not always necessary. At times, proper hemostasis of the bare area can be achieved with argon beam coagulation.
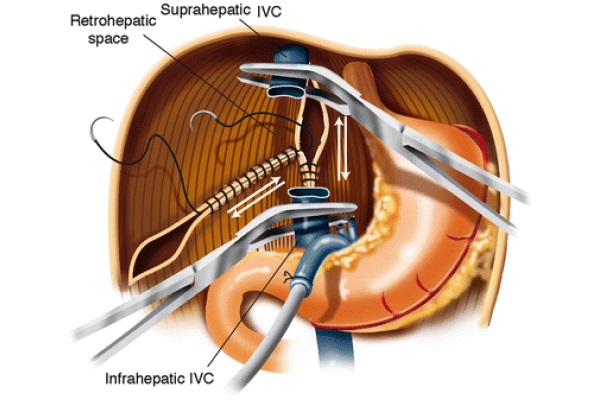
Fig. 3 - Bare area hemostasis
Achieving hemostasis by oversewing the bare area tends to decrease the size of the retro-hepatic area, allowing for transplantation of a smaller liver. This change in size of the right upper quadrant of the abdomen should be kept in mind when using large livers that might not fit the anatomical area. In that case, different hemostatic techniques should be considered.
The cuffs of the supra- and infra-hepatic IVC are prepared, and the new liver is brought into the operative field. It is helpful to position two 4/0 silk sutures on the upper left of the suprahepatic IVC cuff. By pulling these two sutures cranially, a better exposure of the posterior wall of the anastomosis is achieved. The suprahepatic IVC anastomosis is done in an end-to-end fashion using running 3/0 Prolene sutures. Subsequently, the infra-hepatic IVC anastomosis is completed in an end-to-end fashion using running 4/0 Prolene sutures. Once the posterior wall of the infra-hepatic IVC anastomosis is completed, the liver is flushed with 1 l of chilled (4 °C) lactated Ringer’s (LR) solution. The allograft is flushed through a cannula that was secured in the portal vein at the time of the back-table preparation. The practice of flushing the liver with chilled LR intends to remove as much University of Wisconsin® solution (UW) as possible from the allograft. UW is rich in potassium. If the UW is not removed from the allograft, a load of potassium would reach the right atrium at the time of reperfusion. This process may be responsible for deadly cardiac arrhythmias. At this stage, in preparation for the portal vein anastomosis, the portal cannula of the venous-venous bypass is clamped with a tubing clamp. The portal cannula is removed from the portal vein, and the portal vein is clamped with a pediatric angled Potts clamp. The surgeon should clamp the tip of the portal vein cannula with a large Kocher clamp to prevent air embolism in the case of failure of the tubing clamp. In preparation for the portal vein anastomosis, three wet lap sponges are placed between the right hemidiaphragm and the dome of the liver. The right arm of the rib-grip retractor is lowered by three complete turns. The combination of these two maneuvers shortens the distance between the donor and recipient’s portal vein stumps. This prevents the creation of a long portal vein that could kink and therefore cause vessel thrombosis in the postoperative time. The donor’s portal vein is then shortened to a sufficient length to obtain a straight and nonredundant anastomosis. The portal vein anastomosis is completed end-to-end with running 6/0 Prolene sutures. When the running suture is tied, a generous growth factor is left behind so the anastomosis can expand at reperfusion and stenosis can be prevented. The laps are removed from the field and the rib-grip retractor is placed in its original position. There are two ways of proceeding at this time. One way is to perfuse the liver solely with portal blood and to reconstruct the hepatic artery once reasonable hemostasis is achieved. The second option consists of reconstructing all of the vessels before reperfusing the allograft. This second option is the one favored. However, in order to safely proceed with four-vessel reconstruction before reperfusion, the total implantation time cannot exceed 1 h. In addition, reperfusing allografts with portal as well as systemic blood can cause more hemodynamic instability that should be taken in account by the anesthesiology team. The four-vessel technique is completed as follows. The GDA is tied off with two 2/0 silk ties. The use of two separate sutures guarantees better control of this vessel. The common hepatic artery is clamped with a spoon clamp, and the recipient’s hepatic artery is opened. A cuff of the recipient’s hepatic artery is prepared at the level of the GDA patch. The donor’s hepatic artery is shortened and beveled in the opposite direction, and a straight and nonredundant anastomosis is made end-to-end using running 7/0 Prolene sutures. Once the anterior wall of the hepatic artery anastomosis is completed, the anesthesia team is alerted that the allograft will be reperfused in approximately 3 min. This gives the anesthesiologist enough time to prepare the drugs needed to counteract possible hemodynamic instability after reperfusion and to record the last potassium level before reperfusion. At this stage the liver is reperfused. The hepatic artery, the portal vein, the infra-hepatic IVC, and the suprahepatic IVC clamps are removed in sequence. It is possible to keep the suprahepatic IVC clamp on until the liver is fully reperfused. In this case, the liver outflow runs into the bypass machine before reaching the heart rather than going directly into the right atrium. This maneuver prevents a potentially fatal, arrhythmogenic potassium load.
If the patient is stable after packing the operative field, it is suggested to go off bypass. After all cannulas are removed, the blood in the circuit can be recuperated in the cell saver. The next step of the operation is to achieve hemostasis. It is customary to proceed in a clockwise fashion starting from the anterior wall of the suprahepatic IVC, moving toward the medial side wall of the same vessel, to the infra-hepatic IVC on its medial aspect, to the hepatic hilum, and lastly to the lateral walls of the infraand suprahepatic IVC. There are several areas not mentioned above that are addressed while moving in the clockwise direction, namely, the falciform ligament on both sides (donor and recipient), the left triangular ligament on both sides, the hepatogastric ligament on the recipient’s side, and the right triangular ligament on the donor’s side. Generally, these areas are addressed with argon beam coagulation. Packing during hemostasis is exceptionally important because pressure alone has been shown to be one of the most effective methods of achieving hemostasis, and because it maintains hemostasis while the anesthesiologist progressively corrects any coagulopathy based on the results of thromboelastography (Kang et al. 1985).
The last step of the operation is the bile duct reconstruction that will be discussed in a separate paragraph.
This same operation can be performed without venous-venous bypass when the degree of portal hypertension is such that cross-clamping the IVC would not cause a significant reduction in the blood flow return to the right heart. Hemodynamic stability in this case can be achieved by maintaining the patient’s electrolyte and volume status (Starzl et al. 1968).
Bile Duct Reconstruction
The bile duct continuity can be achieved with several techniques. It is preferential to perform an end-to-end choledochocholedochostomy over a T tube. First, the donor’s duct is explored. This is done with a bile duct probe. With this maneuver, the distance between the stump of the donor’s duct and the bifurcation in the right and left duct is assessed. Next, cholecystectomy is carried out in an antegrade fashion using electrocautery. The cystic artery is transected to check for good blood flow from the hepatic artery. The cystic artery is then tied off with a 2/0 silk tie when satisfactory pulsating blood flow is identified. The gallbladder is removed from the surgical field. Hemostasis is achieved in the bed of the gallbladder with argon beam coagulation. The cystic duct is then opened flat with electrocautery to avoid possible mucocele formation in the postoperative time. The stump of the bile duct is trimmed by 1 or 2 mm until arterial bleeding is noted from the edge of the duct. The bleeding vessels are always located in the medial and lateral corner of the bile duct stump; hemostasis is achieved with one transfixed stitch per arterial vessel. Different types of stitches can be used, such as 4/0 silk or 6/0 PDS. At this stage, two 4/0 silk stitches are placed on the lateral and medial corner of the donor’s bile duct stump. The recipient’s duct is opened, explored, and trimmed. Although this anastomosis can be performed with or without a T tube, T tube use is preferred. The T tube gives easy access to the bile duct if a cholangiogram is needed in the postoperative time. Other uses of the T tube include: macroscopic evaluation of the bile characteristics and bile collection for culture in case of postoperative infections. Thick dark bile is considered to be normal. Bile that is light in color, and less dense than normal, is typical in primary non-function or acute cellular rejection. Nine 5/0 PDS sutures are used to complete the anastomosis. Two stitches are placed in the posterior wall, three on each side, and one on the anterior wall. A purse string is placed around the exit site of the T tube. Lastly, the anastomosis is checked for leakage by injecting heparinized saline solution through the T tube first, and air, while the anastomosis is submerged in water, second.
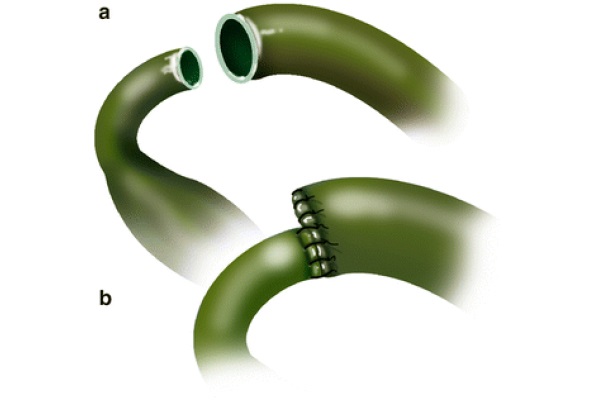
In cases of significant size discrepancy between the donor and recipient’s ducts, the larger duct is partially sutured closed prior to performing the anastomosis, as displayed in Fig. 4 (Busuttil and Klintlalm 1996). Alternatively, a choledochojejunostomy over a Roux-en-Y can be used for the same purpose (Sarmiento 2000). The latter is also indicated for patients with primary sclerosing cholangitis (PSC), a disease that carries an increased risk of malignancies of the bile duct. From a technical standpoint, the Roux-en-Y loop is created in the usual fashion, using a hand-sewn technique in two layers, where the sero-serosa is completed with 4/0 silk interrupted sutures. The inner layer is anastomosed using the Gambee technique with 4/0 PDS. The tip of the afferent loop is oversewn with interrupted 4/0 silk stitches. The Roux loop is placed ante-colic; however, a retrocolic placement is an acceptable choice. The ante-colic option is safer, because although portal hypertension is resolved after reperfusion, enlarged varicosities are spread through the abdominal cavity, including the transverse mesocolon. These varices can be injured when the tunnel in the transverse mesocolon is created. Once the intestine is opened, in the antimesenteric border, the mucosa and the serosa are tucked together in the four cardinal points using 6/0 PDS. A silastic tube, placed across the anastomosis, is anchored to the intestine with 5/0 chromic; the 9 stitches technique previously described is used to complete the choledochojejunostomy.
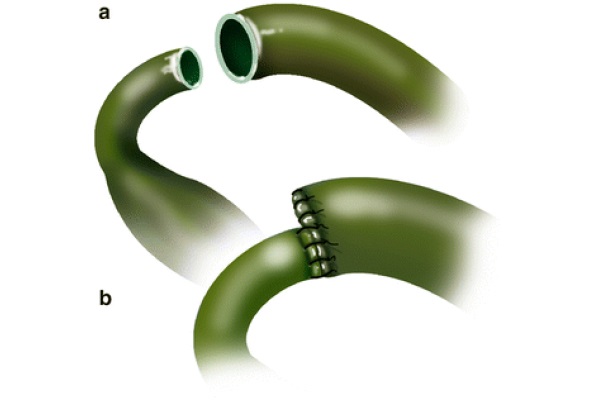
Fig. 4 - End-to-end choledococholedochostomy without T tube
Piggy-Back Technique
There are different ways of performing a liver transplantation using the piggy-back technique. The common denominator of the different approaches is that the recipient’s caval blood flow to the heart is never more than partially occluded at any time during the surgery. Uninterrupted flow through the IVC affords ample venous blood flow, proper cardiac filling, and hemodynamic stability throughout the procedure (Calne and Williams 1968). Sir Roy Y. Calne first described the piggy-back technique, but Andreas Tzakis popularized it in 1989 (Tzakis et al. 1989). It is especially indicated in cases when the donor’s liver is smaller than the diseased liver (Tzakis et al. 1989). The procedure is identical to the standard technique up until the skeletonization of the hepatic hilum is completed.
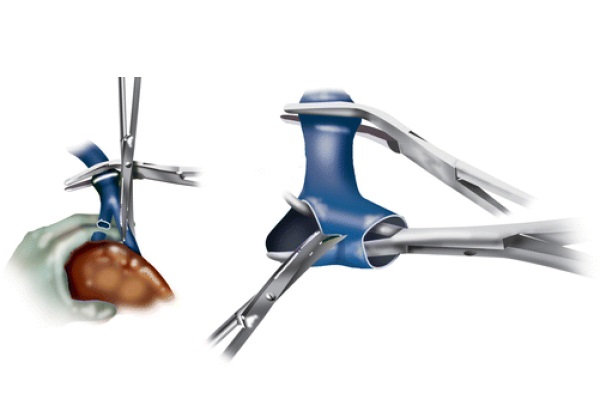
Subsequently, the liver is peeled off the IVC. The accessory hepatic veins are divided between 2/0 silk ties, and the accessory hepatic caval stumps are sutured with transfixed 4/0 silk stitches. This is done to prevent the ties from coming off in the postoperative time due to the swings in the central venous pressure (CVP), which are typical during recovery in the intensive care unit (ICU). The right hepatic vein is skeletonized free and clamped both proximally and distally. It is subsequently divided, and the two stumps are oversewn with running 4/0 Prolene sutures. The portal vein is divided between clamps. A German clamp is applied at the common trunk of the left and middle hepatic veins anterior to the vena cava, and the liver is removed from the surgical field using blunt and sharp dissection. Figure 5 demonstrates the left and middle hepatic veins being opened, and the septa separating both veins being cut, creating a common cuff.
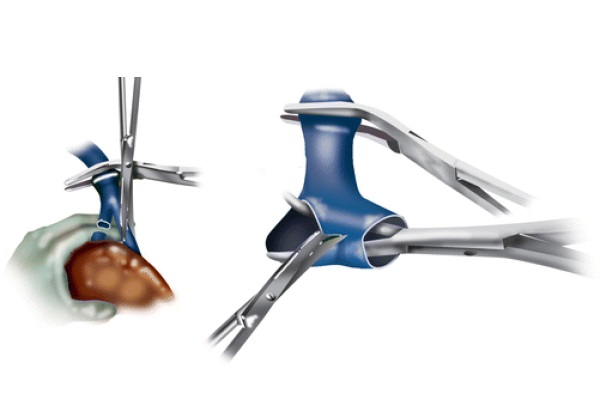
Fig. 5 - Left and middle hepatic vein division and cuff formation
The new liver is brought into the field. The anastomosis between the recipient’s hepatic venous cuff and the donor’s suprahepatic IVC is completed in an end-to-end fashion using 4/0 Prolene sutures. In this case, the upper left of the hepatic veins cuff is kept open by two 4/0 silk stitches while the posterior wall is sewn. Once the posterior wall of this anastomosis is completed, the liver is flushed with 1 l of chilled LR at 4 °C. Subsequently, the allograft’s infra-hepatic IVC is tied off. The portal vein and hepatic artery anastomoses are performed as in the standard technique. At this stage, the liver is reperfused. The hepatic artery, the portal vein, and the hepatic vein clamps are removed in sequence. In the piggy-back procedure as well, the liver reperfusion can be done with or without hepatic arterial flow. After a few complete rounds of hemostasis, the bile duct reconstruction is completed as previously described. More hemostasis is performed, and the field is washed with several liters of antibiotic solution. Three Jackson-Pratt drainages are place in the abdominal cavity.
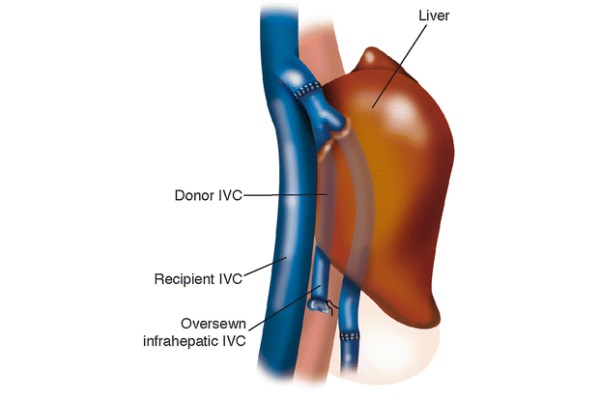
Since there is no end-to-end infra-hepatic IVC anastomosis, the completed procedure leaves the recipient with a section of the donor’s IVC lying anteriorly to the recipient’s IVC, as diagrammed in Fig. 6. Within 1 week post-operation, a thrombus is often visible by ultrasound in the section of the donor’s IVC sandwiched between the liver and the recipient’s IVC. This is common and is not pathologic, as it does not often result in pulmonary embolus (Tzakis et al. 1989). There is no reason to administer anticoagulant medications in this case.
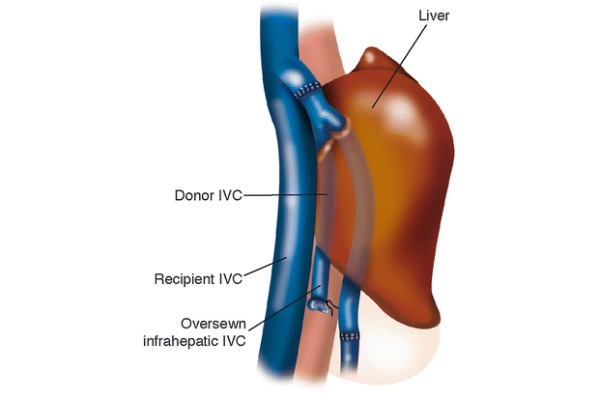
Fig. 6 - Anatomy post piggy-back procedure
Piggy-Back with Side-to-Side Caval Anastomosis (Also Known as the Belghiti Technique)
Jacques Belghiti described the side-to-side piggy-back technique. Without removal of the retro-hepatic vena cava, and by requiring only one caval anastomosis, Belghiti claimed to reduce the duration of the anhepatic phase (Belghiti et al. 1992). It is the opinion of the authors that this is not the case. Belghiti describes that often, the piggy-back procedure necessitates temporary caval occlusion when creating the common cuff of the left and middle hepatic veins. This assumption is arguable, especially because in the classic piggy-back technique, the common trunk of the middle and left hepatic veins is clamped, a structure that is distant from the IVC. In the Belghiti technique, the IVC is always partially clamped.
In the following paragraph, discussion of the portions of the operation that are not different form the ones previously discussed is omitted.
The IVC is peeled off retro-hepatically by dividing the accessory hepatic veins between 2/0 silk ties.
Following this, the left, middle, and right hepatic veins are clamped, divided, and oversewn. The vast majority of the surgeons embracing this technique perform this part of the operation with mechanical staplers, increasing the cost of the operation significantly. The liver is removed from the peritoneal cavity, leaving the full length of the IVC intact.
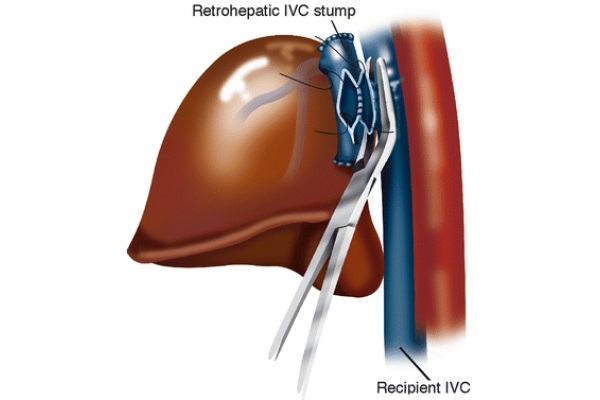
To perform the cavotomy on the recipient’s IVC, a vascular clamp is placed on a section of the IVC “pinching” the vessel’ side wall, as exhibited in Fig. 7. This incomplete vascular clamp ensures a constant caval flow throughout the procedure. This clamp stays on the recipient vessel until the surgeon is prepared to allow portal blood to flow into the IVC. The donor’s cavotomy is performed on the back table as well as oversewing of the donor’s supra- and infra-hepatic cavas. This ensures that once the caval anastomosis is performed, blood flows from the donor’s IVC stump into the recipient’s IVC. The donor’s liver is then brought into the peritoneal cavity, and a side-to-side caval anastomosis is created, connecting the donor and recipient’s retro-hepatic vena cavas. The vessels of the porta hepatis are anastomosed. Once the portal vein anastomosis is complete, both portal and caval clamps are removed. This is, generally speaking, done in sequence while the running suture on the caval anastomosis is not tied off yet, so that the residual air in the anastomosed vessels can vent out of the blood stream. Supposedly, an advantage of this technique is that the anastomosis formed between the donor and recipient’s vena cavas is large. This ensures that there will not be outflow obstruction.
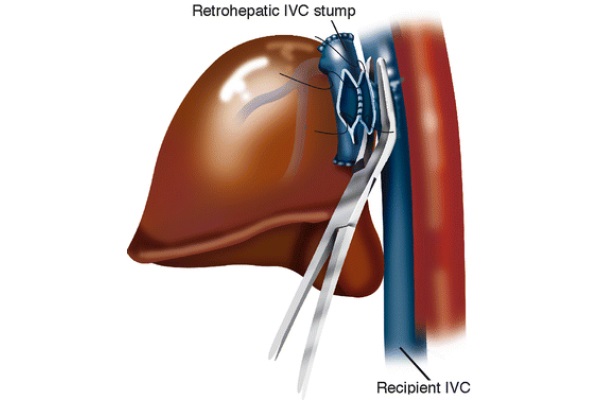
Fig. 7 - Belghiti modification of the piggy-back procedure
Although this procedure technically is feasible, there are several drawbacks. Following this procedure, there tends to be a large amount of scar tissue formation postoperatively surrounding the side-to-side caval anastomosis. In the event that the patient needs a secondary liver transplant, it is difficult to access the suprahepatic IVC to remove the existing liver. Also, due to the nature of the neo-anatomy, if a TIPS procedure is needed, it is difficult to reach the right hepatic vein from the internal jugular vein, because with the new anatomy, a near right angle is formed at the IVC anastomosis.
Liver Transplantation in Situs Viscerus Inversus
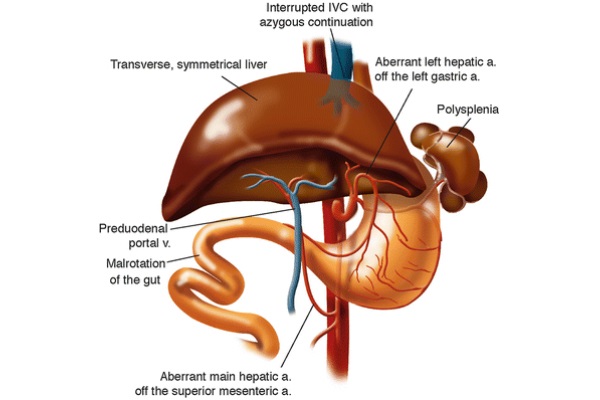
Situs inversus (SI) is a rare congenital condition in which the abdominal viscera are located in mirror image positions across the midline compared to situs solitus. The inferior vena cava is located to the left of the abdominal aorta. During normal embryological development, the liver forms a larger right lobe compared to the left lobe due to asymmetric venous outflow (Farmer and Busuttil 1996). In SI, asymmetric hepatic venous outflow does not exist, leading to the formation of a symmetric liver. Other complications, presented in Fig. 8, often coexist with SI including polysplenia, aberrant left hepatic artery, aberrant main hepatic artery, preduodenal portal vein, malrotation of the gut, and interrupted IVC with azygous continuation (Farmer and Busuttil 1996).
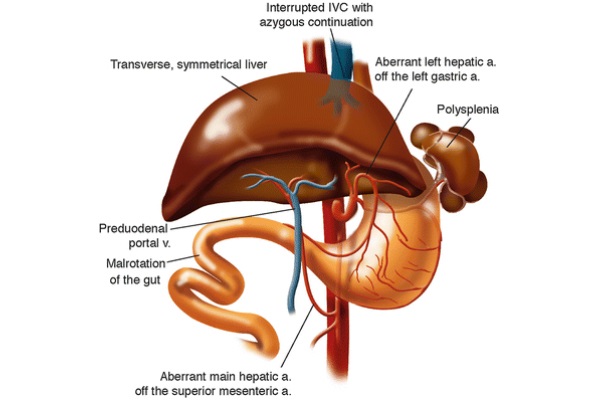
Fig. 8 - Common complications with situs inversus
Indications for transplant in SI patients are similar to those with SS anatomy. Often, the discovery of SI anatomy occurs during radio-imaging for nonrelevant procedures. There is much debate on how to position the allograft in a patient diagnosed with SI who needs transplantation. If a SS allograft is positioned without rotation in the recipient’s upper left quadrant, the large right hepatic lobe lays across the stomach and vertebral column where space is restricted.
Dr. Klintmalm and associates described a case, in 1991, of a liver transplantation into an SI patient (Klintmalm et al. 1993). All vessels and ligaments to the recipient liver are divided, and the retrohepatic IVC is left intact. The native liver is removed, and the allograft, in preparation for the implantation, is rotated clockwise 90°, as in Fig. 9, with the left lobe pointing into the left iliac fossa and the right lobe sitting in the recipient’s hepatic fossa. The donor’s suprahepatic vena cava is oversewn. The donor’s infra-hepatic vena cava is anastomosed end-to-side to the recipient’s left-sided inferior vena cava. The other major vessels are anastomosed end-to-end and blood flow to the allograft is restored. Benefits of this technique are the recipient’s stomach is not obstructed by the donor’s right lobe, there is no risk of venous outflow obstruction, and the size of the allograft is not an exceedingly important factor (Klintmalm et al. 1993).
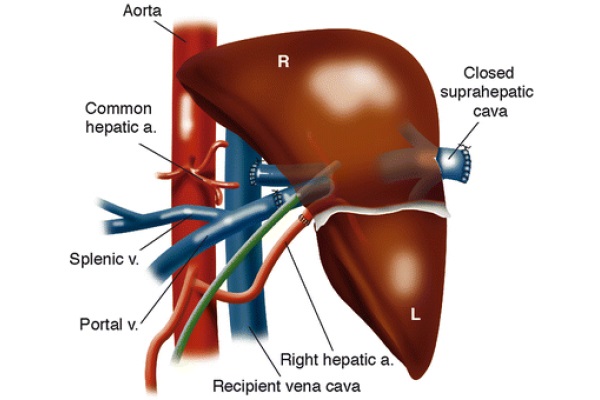
Fig. 9 - Situs inversus anastomoses
Auxiliary Liver Transplantation
Orthotopic liver transplant (OLT) is the treatment of choice for fulminant hepatic failure (FHF) (Bismuth et al. 1995). A suitable alternative is auxiliary liver transplantation, a procedure where the allograft is transplanted either heterotopically or orthotopically in place of a section of the native liver. The allograft is removed after the native liver recovers from the original insult. The main advantage of this procedure is that the patient avoids life-long immunosuppressive treatment once the allograft is removed (Jaeck et al. 2002). The theory behind auxiliary liver transplant is that the implanted allograft will lend the patient hepatic support while the failing liver regenerates. Orthotopic versus heterotopic auxiliary partial liver transplant is debated.
Auxiliary partial orthotopic liver transplantation (APOLT) is completed by removing a section of the failing liver and replacing it with an equal lobe or part of a lobe from a donor. This operation is more commonly done in children using a live donor. Depending on the recipient’s size, the left lobe or the left lateral segment is used (Jaeck et al. 2002). When the left lateral segment is chosen, the donor’s operation requires the resection of the left lateral segment with accompanying vessels. The donor’s vessels procured with the partial allograft consist of the stumps of the left portal vein, hepatic artery, and hepatic vein. The left bile duct is taken with the allograft.
The allograft’s left hepatic vein is anastomosed end-to-end with the recipient’s vessel. The donor’s hepatic artery is anastomosed, preferably, with the recipient’s left hepatic artery. The portal vein and the bile ducts are anastomosed to their corresponding recipient vessels. The anatomy of the recipient right liver is left untouched. The hepatic function offered by the partial allograft provides the failing liver time to heal. Primary non-function of the graft is not statistically different between OLT and APOLT (van Hoek et al. 1999).
Gubernatis et al. explain that hypothetically, portal blood flow should be distributed between the two livers by resistance to flow. Early after transplantation, blood should preferentially flow through the allograft due the high resistance in the diseased liver. Once the diseased liver begins to heal, blood flow shifts back to the healing liver due to resistance in the donor allograft. This increased resistance is the result of rejection, once the immunosuppressive medication is discontinued. Conveniently, the blood flow is preferentially distributed to the healthy liver section (Gubernatis et al. 1991).
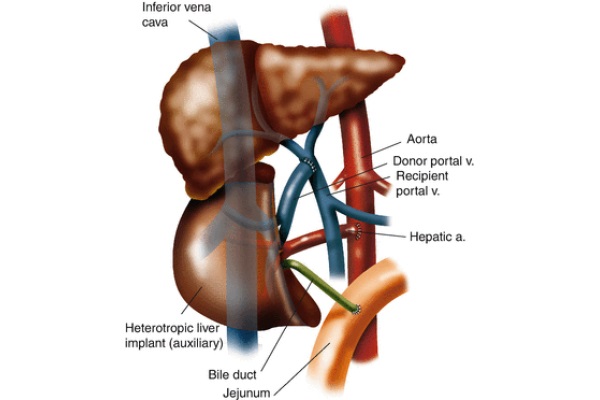
Fig. 10 - Heterotopic auxiliary partial liver transplant anastomoses
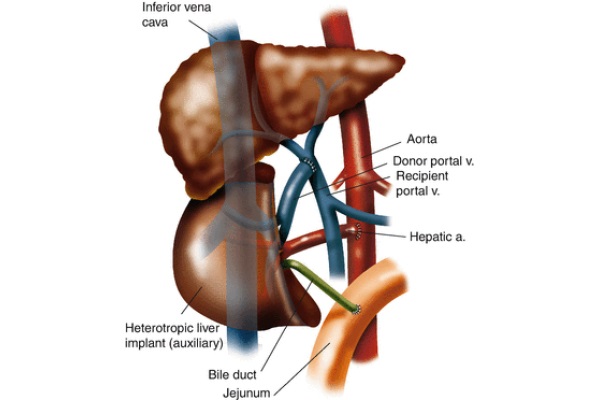
Heterotopic auxiliary partial liver transplant is a procedure where the recipient’s liver is left intact while a partial donor allograft is implanted into the recipient’s subhepatic space as illustrated in Fig. 10. The goal of this procedure is the same as APOLT, but the efficacy is diminished (van Hoek et al. 1999). This operation can be accomplished using a cadaver or a live donor. In preparation for the allograft’s implantation when a cadaver donor is used, a section of the allograft is resected so it can be accommodated in a small space. On the back table, the suprahepatic IVC is oversewn. Upon implantation, an end-to-side anastomosis of the donor’s infra-hepatic IVC and the recipient’s suprarenal IVC is performed. Anastomoses are created between the recipient’s celiac axis and the donor’s hepatic artery as well as the donor and recipient’s portal veins. An end-to-side choledochojejunostomy is performed between the donor’s bile duct and the recipient’s jejunum. Although the heterotopic implantation of a partial liver should be technically performed as well as APOLT, it does not due to issues such as elevated venous backpressure and inadequate portal perfusion of the donor and recipient’s partial livers (Gubernatis et al. 1991).
Conclusion
In a span of 50 years, liver transplantation has evolved dramatically and faster than any other known field in medicine. This is partially due to a better understanding of the physiology of patients with end-stage liver disease and the intraoperative management of these patients. Furthermore, the introduction of the venous-venous bypass and the development of structured training programs have made this procedure a routine. It is unfortunate that we have not observed any growth in transplantation in the past several years despite a continuous increase in the number of patients waiting for a liver transplant. As a consequence of that, access to liver transplantation is still restricted to less than 150 centers in the entire United States. It should not be forgotten that although routine, when compared with any other field in surgery, liver transplantation is still a procedure left in the hands of a very limited number of extraordinarily talented surgeons committed to serving a group of patients that for the most part fall into the category of the “underprivileged.”
All images are courtesy of Paul Schiffmacher, Medical Illustrator for Medical Media Services at Thomas Jefferson University.
References
Belghiti J, Panis Y, Sauvanet A, Gayet B, Fekete F (1992) A new technique of side to side caval anastomosis during orthotopic hepatic transplantation without inferior vena caval occlusion. Surg Gynecol Obstet 175(3):270–272
PubMed
Bismuth H, Samuel D, Castaing D et al (1995) Orthotopic liver transplantation in fulminant and subfulminant hepatitis. The Paul Brousse experience. Ann Surg 222(2):109–119
CrossRef PubMed PubMedCentral
Busuttil RW, Klintlalm GB (1996) The recipient hepatectomy and grafting. In: Transplantation of the liver, 1st edn. W.B. Saunders, Philadelphia, pp 405–418
Calne RY, Williams R (1968) Liver transplantation in man – I, observations on technique and organization in five cases. Brit Med J 4(535):540–652
CrossRef PubMedCentral
Farmer DG, Busuttil RW (1996) Liver transplantation and situs inversus. In: Transplantation of the liver, 1st edn. W.B. Saunders, Philadelphia, pp 474–480
Gubernatis G, Pichlmayr R, Kemnitz J, Gratz K (1991) Auxiliary partial orthotopic liver transplantation (APOLT) for fulminant hepatic failure: first successful case report. World J Surg 15(5):660–665 (discussion 665–666)
CrossRef PubMed
Jaeck D, Boudjema K, Audet M et al (2002) Auxiliary partial orthotopic liver transplantation (APOLT) in the treatment of acute liver failure. J Gastroenterol 37(Suppl 13):88–91
CrossRef PubMed
Kang YG, Martin DJ, Marquez J et al (1985) Intraoperative changes in blood coagulation and thrombelastographic monitoring in liver transplantation. Anesth Analg 64(9):888–896
CrossRef PubMed PubMedCentral
Klintmalm GB, Bell MS, Husberg BS et al (1993) Liver transplant in complete situs inversus: a case report. Surgery 114(1):102–106
PubMed
Sarmiento JM (2000) Hepaticojejunostomy: Indications and Surgical Technique. Operative Techniques
in General Surgery 2(4):295–303
CrossRef
Shaw BW Jr, Martin DJ, Marquez JM et al (1984) Venous bypass in clinical liver transplantation. Ann Surg 200(4):524–534
CrossRef PubMed PubMedCentral
Starzl T (1969) Experience in hepatic transplantation. W.B. Saunders, Philadelphia
Starzl TE, Groth CG, Brettschneider L et al (1968) Orthotopic homotransplantation of the human liver. Ann Surg 168(3):392–415
CrossRef PubMed PubMedCentral
Tzakis A, Todo S, Starzl TE (1989) Orthotopic liver transplantation with preservation of the inferior vena cava. Ann Surg 210(5):649–652
CrossRef PubMed PubMedCentral
van Hoek B, de Boer J, Boudjema K et al (1999) Auxiliary versus orthotopic liver transplantation for
acute liver failure. J Hepatol 30(4):699–705
CrossRef PubMed
 06.60301809
06.60301809